The landscape of medical research is ever-evolving, with breakthroughs continuously shaping our understanding and treatment of diseases. One area witnessing transformative advancements is small animal imaging, an integral part of preclinical research. A leap forward has been made with the introduction by TomoWave of the Laser Optoacoustic Imaging System (LOIS-3D) to the global market.
Revamping Traditional Imaging Paradigms
Traditionally, imaging systems like X-ray, MRI, and ultrasound have contributed significantly to research on mouse models of diseases. However, these systems mainly provide anatomical images, lacking critical functional information on blood content, oxygen saturation, and the molecular composition of tissues.
The Breakthrough: TomoWave Optoacoustic Imaging
Introducing LOIS-3D, a game-changer employing full-view three-dimensional quantitative Optoacoustic Tomography (qOAT). This innovative technology employs the most compelling properties of light and sound, delivering three-dimensional images of small animal models of huma diseases with unprecedented detail (spatial resolution of 150 micron equal in all 3 dimensions) and exceptional contrast (the highest sensitivity to changes in the optical absorption of tissue on the market allowing detection of ~1 pM concentrations of highly absorbing gold and silver nanoparticles). For kinetic measurements of drug and molecular contrast agent distribution in the mouse body and brain, the most advanced version of LOIS-3D offers temporal resolution of 5 sec per 3D volumetric image acquisition.
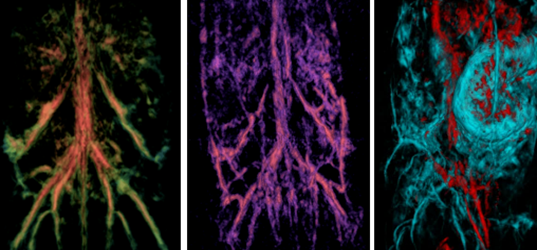
How Does It Work?
The method of depth-resolved Optoacoustic/Photoacoustic Imaging invented by TomoWave founder, Dr. Alexander Oraevsky in 1993, takes advantage of (i) the strong optical absorption in biological tissues provided by molecules of hemoglobin and oxyhemoglobin within red blood cells in the near-infrared spectral range where optical scattering is much weaker than in the visible spectral range allowing effective penetration of the optical energy into the depth of tissues, (ii) the effect of the light conversion into the sound (discovered by Alexander Graham Bell in 1880), and (iii) the delivery of NIR light by short (nanosecond) LASER pulses (first proposed by Gordon Gould in 1958 and called Q-switching). Delivery of the optical energy into the tissue under conditions of temporal pressure confinement within the voxel to be resolved enables high spatial resolution of optoacoustic images despite the optical scattering that blurry pure optical images. Thus, “seeing” through the body by “listening” to the sound generated by light becomes possible, offering doctors and biomedical researchers an opportunity detect cancer, stroke and peripheral vascular diseases, visualize arteries and nerves helping surgeons to avoid accidental cuts, revealing blood content, oxygen saturation of tumors, measuring biodistribution of molecular contrast agents, and other critical parameters that traditional imaging systems don’t capture.
Impact on Preclinical Research
TomoWave Imaging systems offer comprehensive view of mouse models of diseases in the body and in the brain, enriching preclinical research. Researchers can now investigate tissues with higher precision, enhancing the understanding of disease mechanisms and paving the way for next-generation therapies.
A Look into the Future of Small Animal Imaging
With TomoWave, the era of small animal imaging is entering an exciting phase, promising further breakthroughs in medical research. The combination of light and sound in imaging has opened doors to new possibilities, ushering in a future where diagnosing and treating diseases will be more accurate, safe and effective. In the end of 2023, TomoWave will launch a new advanced Animal Research Imaging Assembly (ARIA) for preclinical imaging enhancing performance of the full-view 3D optoacoustic tomography system with real time imaging capability using high-end ultrasound plus optoacoustic hand-held probes.
In conclusion, the advent of TomoWave optoacoustic tomography systems represents a remarkable stride in small animal imaging. By providing comprehensive, three-dimensional images of tissues, it is revolutionizing preclinical research and catapulting it into the future of clinical imaging. As we continue to unravel its potential, there is no doubt that this technology will be instrumental in transforming healthcare and improving human lives.
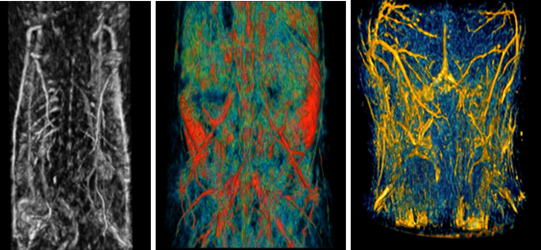
Wow, this paragraph is good, my younger sister is analyzing
such things, therefore I am going to let know her.
Greetings! I’ve been reading your blog for a while now and finally got the
bravery to go ahead and give you a shout out from Huffman Tx!
Just wanted to tell you keep up the good work!
Thankks for ones marvlous posting! I genuinely enjoyed reading it,
you might be a great author.I will make certain to bookmark your blog and may come back very soon. I want to encourage
you to continue your great writing, have a nice day!
Look into my web page … https://Ukrain-Forum.BIZ.Ua